2024 Important Performance
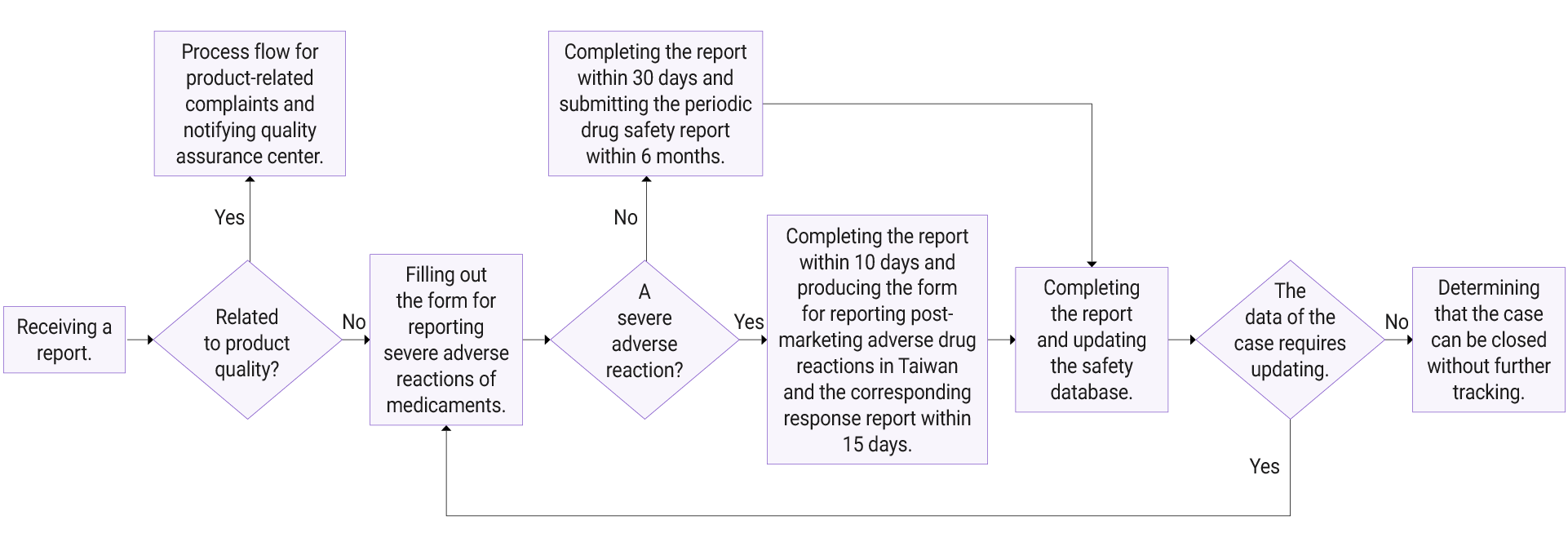
Oneness Biotech has created a “Pharmacovigilance System” according to the Pharmaceutical Affairs Act, the Regulations for the Management of Drug Safety Surveillance, the Regulations for Reporting Serious Adverse Drug Reactions, the Guidelines for Filling Out Forms for Reporting Serious Adverse Drug Reactions, the ICH Guideline E2C (R2) on Periodic Benefit-Risk Evaluation Report (PBRER), and so on. The company established the Post-Marketing Drug Quality Monitoring System, which, due to organizational restructuring in September 2023, is now led and coordinated by the Regulatory Affairs Team under the President’s Office. The Quality Assurance Center, the R&D, sales, clinical, and IT departments collaborate to ensure that the system is in normal operation and that all the necessary documents are prepared, archived, and reported as required. During the reporting period of 2021-2024, no serious adverse drug reactions were reported.
Oneness Biotech collects cases of adverse drug reactions through the monitoring system, has created and maintains a report database, and keeps monitoring the safety of the approved drugs, in order to protect patients’ safety and take on responsibilities for its products and to patients using the products.
Sales activities of medicinal drugs in line with WHO Ethical criteria for medicinal drug promotion
Oneness Biotech has formulated the “Codes of Ethical Conduct” and “Marketing and Sales Code of Conduct”. It is required that marketing and sales personnel must comply with relevant laws and regulations and the recognized ethical standards of the pharmaceutical industry. Marketing documents must be internal reviewed to ensure the content is consistent with the indications and in compliance with regulatory requirements. The Company regularly (quarterly/yearly) organizes education and training to educate relevant personnel to sell medicines properly; and shares medical information with medical service providers and patients in an open, transparent, and timely manner to avoid information asymmetry. There was no violation noted in 2024.
Internal Audit and External Verification
To ensure the effectiveness and compliance of its quality management system, the Company adopts a dual mechanism of internal audits and third-party certifications. Through both scheduled and unscheduled audits, we ensure that all departments comply with standard operating procedures (SOPs) and proactively identify and address potential risks.
At least two internal audits are conducted annually, covering key departments such as production, quality assurance (QA), quality control (QC), and warehousing. Departments must implement corrective actions within 30 days for any deficiencies found, while the Quality Management Department tracks progress.
According to the 2024 internal self-inspection plan, the following internal audits were completed:
※The above content is taken from the ESG Report