The Company has established the “Risk Management Policies and Procedures” as the highest guiding principle for risk management.
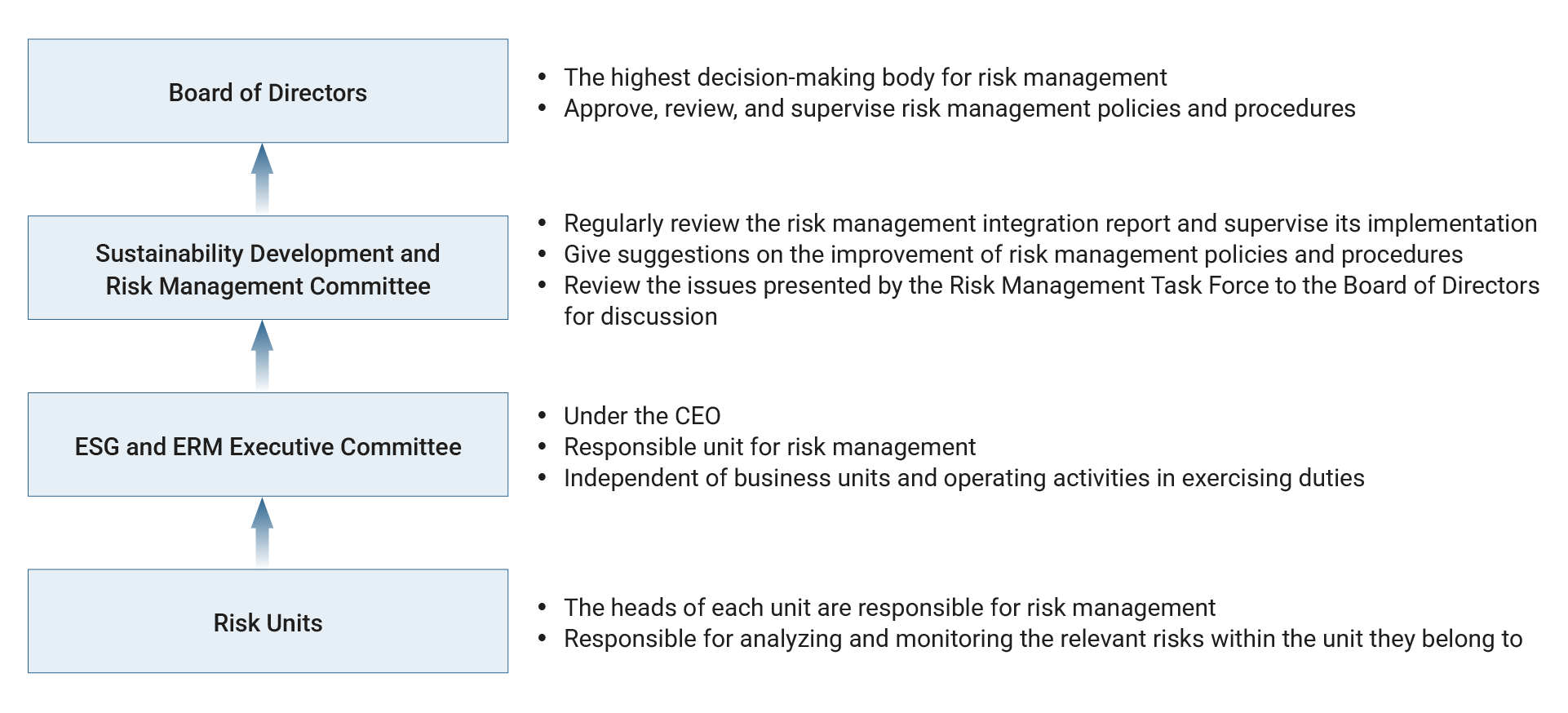
The Board of Directors is the highest decision-making body for the risk management. It is responsible for approving, reviewing, and supervising the Company’s risk management policies, ensuring the effectiveness of risk management, and bearing the ultimate responsibility. The Board of Directors has established the “Sustainability Development and Risk Management Committee”, whose members are all independent directors.
The ESG and ERM Executive Committee is the unit responsible for executing the Company’s risk management. Its primary duties include monitoring, measuring, and assessing of the Company’s risks. It shall exercise its duties independent from business units and operational activities. Starting in 2024, the Executive Committee reports at least twice a year to the Sustainability Development and Risk Management Committee and the Board of Directors. At the beginning of each year, it reports the significant risks for the year and the corresponding response measures, while at the end of the year, it presents the outcomes of risk management execution. For 2025, the reporting dates are February 27 and November 10.



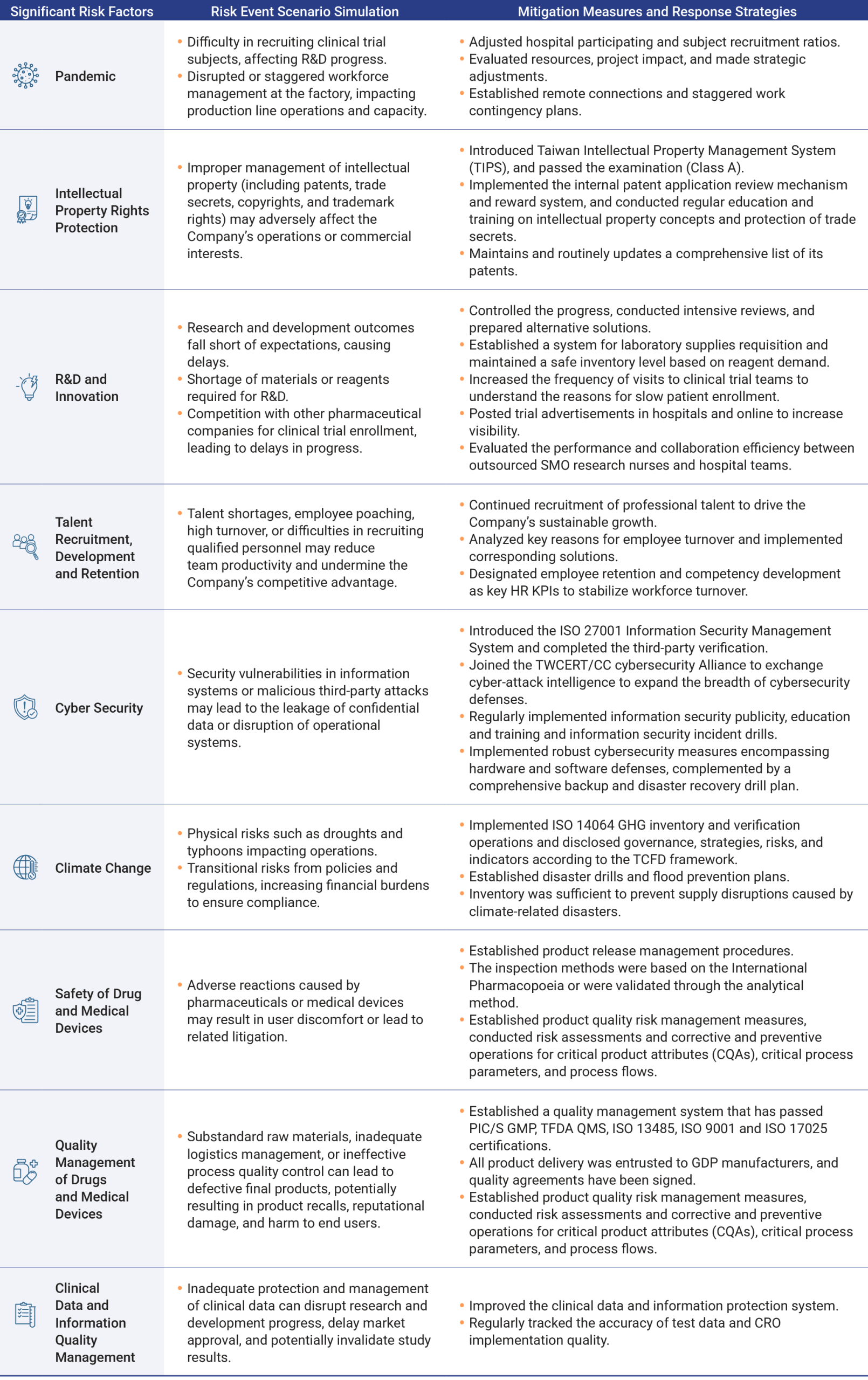
Each relevant unit implemented risk management measures according to the established response strategies. Throughout the year, none of the identified risks had a significant impact on the Company’s operations. The results of the risk management execution were reported to the Sustainability Development and Risk Management Committee and the Board of Directors on November 10, 2025.
In order to strengthen the management, control, and response to future risks, the Company not only predicts the aforementioned risks based on past experience, but also refers to literature published by domestic and foreign institutions to assess emerging risks to understand their possible impacts and formulate countermeasures.
The political situation worldwide has become unstable in recent years. The new US government initiated a trade war based on “Economic Nationalism”, imposing high tariffs on imported products in an attempt to protect its domestic industries, and these tariffs may include pharmaceutical products. If the US government implements drug import tariffs or non-tariff barriers in the future, it may have a significant impact on the Company’s export sales to the US market. Such policy change is out of our control and unpredictable, and its impact can be long-term and significant once it becomes effective. The potential impacts include:
The US market is one of the major markets targeted by the Company. If high tariffs are imposed on drugs, the Company’s price competitiveness for drugs exported to the US will decrease, and the Company may need to absorb some of the tariffs or adjust the product selling price.
High tariffs may result in a decline in sales volume in the US market, which in turn can affect the operating revenue and profit.
It may be necessary to reconsider the supply chain planning (e.g., setting up a factory in the US) or diversify the risk by entering other markets.
Such risk has a high degree of uncertainty, and the risk mitigation measures established by the Company include:
▲ Monitor policy changes continuously: Regularly track US trade policies and international status changes, in particular import and export measures related to the pharmaceutical industry.
▲ Diversify market planning: Actively explore other markets (e.g., Europe, Africa, Asia, etc.) to reduce the dependence on one single market.
▲ Supply chain flexibility: Engage in discussions and negotiations with overseas OEM factories to shift API production abroad, or through investment and cooperation, engage in acquisitions and mergers of existing local channels, to strengthen market presence and supply chain integration, and to adopt such methods as alternative solutions to sudden tariff changes.
Over the past years, the pharmaceutical industry has applied complex artificial intelligence models to analyze the mechanisms of diseases, in order to facilitate the understanding of potential diseases. The rapid development of Generative AI (GenAI) has changed the traditional R&D of drugs and application model of the pharmaceutical industry. The Company also understands GenAI’s ability to automate research, improve clinical trials, and reduce errors, can create benefits for the Company. However, we have also observed the emerging risks associated with GenAI:
The introduction of GenAI into new drug discovery and R&D may present AI technology challenges, such as algorithmic instability or lack of interpretability. In addition, the Company also needs talents equipped with professional knowledge of AI and new drug R&D at the same time, and such talents may be scarce in a short time.
The use of AI technology in new drug R&D may have an impact on the market competition. In particular, five new drugs in the Company’s pipeline are still in the clinical and pre-clinical stages. Competitors may develop drug molecules or antibodies of similar functions at a faster speed, causing the Company’s products to face the pressure of being a “fast-follower”.
The collection and commercialization of patient data (including genetic and social media information) may raise privacy and ethical concerns, and improper use of AI may cause risk to the Company’s reputation.
The extensive application of GenAI offers significant potential for the pharmaceutical industry; however, it remains crucial to further differentiate between reality and hype. Such risk has a high degree of uncertainty, and the risk mitigation measures established by the Company include:
▲ Invest in talent development and recruitment to ensure that the Company has sufficient professional knowledge and technologies.
▲ Establish partnerships with higher academic institutions and research institutions to stay abreast of new technologies.
▲ Maintain sensitivity to the market and competitive environment, and adjust strategies and business models in a timely manner.
▲ Strengthen cybersecurity and privacy management.
※The above content is taken from the ESG Report